凌越生醫過敏原檢測技術平台|高準確度微陣列蛋白質晶片
過敏管理專家 凌越生醫(EBS) 能夠提供高準確度且臨床可信賴的過敏原檢測結果,源自於對 過敏原蛋白原料製備、微陣列蛋白質晶片建置、螢光檢測技術與品質管理系統(QMS) 的完整技術掌握。
自原料來源鑑定、蛋白純化流程、載台選擇,到晶片點片與螢光偵測,各項作業均依循標準化流程並通過多重品質控管,以確保檢測的穩定性、靈敏度與臨床再現性。
以下影片呈現過敏原檢測平台的核心流程,包括 血液檢體處理、蛋白質晶片反應、訊號擷取與數據分析 等關鍵步驟。
▶凌越生醫(EBS)過敏原檢測技術平台揭密影片 (連結)
—原料製備(Preparation)—
A. 過敏原蛋白純化技術
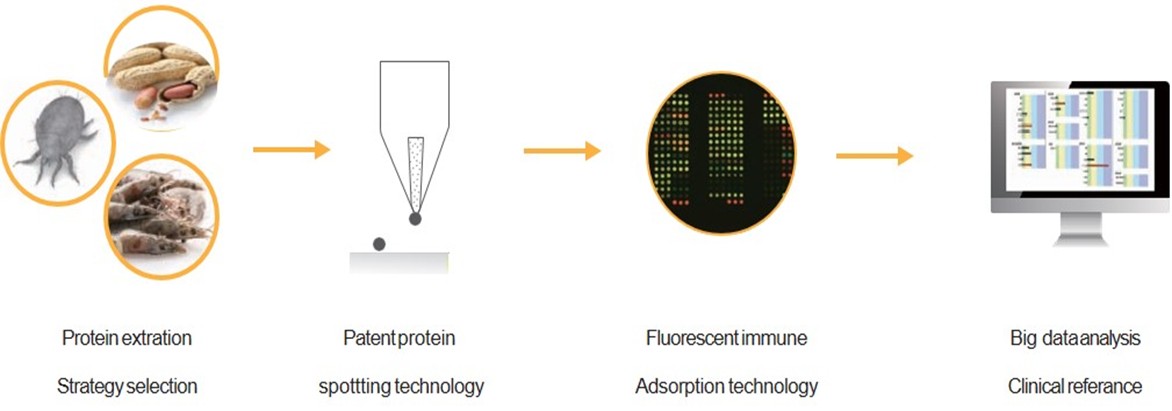
凌越生醫可依當地飲食習慣或所接觸之環境進行客製化,推出不同的蛋白質抗原組合,並搭配不同的蛋白質特性開發出不同的純化萃取流程,製備出高品質的全蛋白抗原原料,並貼近人們的生活習慣。
B. 過敏原蛋白原料品質把關
穩定的過敏原檢測試劑取決於過敏原蛋白原料的穩定性,凌越生醫建立完整的品質管理流程確保每批原料符合高品質要求:
-
安全可靠的原料來源:過敏原原料主要供應商為具國際認證之供應商,同時凌越生醫也自行萃取過敏原蛋白原料,取自天然食材的來源,並逐批進行物種鑑定,確認其來源與品系的正確性。
-
原料穩定性:每批次萃取純化的過敏原蛋白原料都會進行蛋白質定量與蛋白組成確認(SDS-PAGE; Sodium dodecyl sulfate polyacrylamide gel electrophoresis),確保每批原料的濃度及蛋白組成的一致性。
-
過敏原活性確效評估:凌越生醫擁有自己的血清庫及索引系統資訊,利用標準血清庫對陽性品項進行活性測試,確保每批次萃取純化出的過敏原蛋白具有穩定且高效的活性。
C. 載台評估
微陣列技術的載體選擇對檢測系統的穩定性及靈敏度至關重要,凌越生醫在研發過程中將載體特性與過敏原檢測需求進行緊密結合,針對不同蛋白特性,測試多種高品質的玻璃載片選項,如高親水性、低背景訊號或具特殊功能性塗層的載片,最終選定最適合的條件確保過敏原在載體上的均勻分佈及反應效率最佳化。
—蛋白微陣列技術(Microarray protein chip)—
A. 微陣列商品化
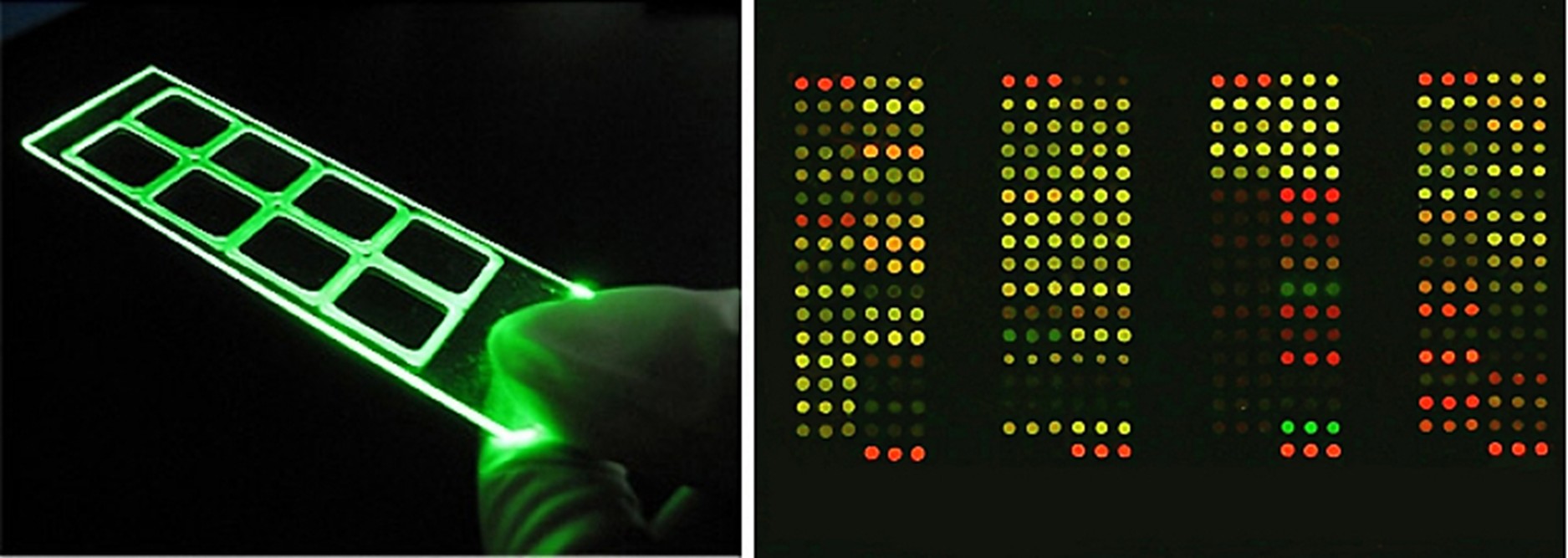
利用微型化抗原點製技術將萃取的抗原精準的點至玻璃載體上;利用抗原抗體專一性結合的特性,結合螢光免疫吸附技術將血清中的生化訊息經雷射激發螢光後,蒐集螢光訊號呈現的強弱進行分析,而後將數據轉化為具臨床意義的半定量參考報告。
.jpg)
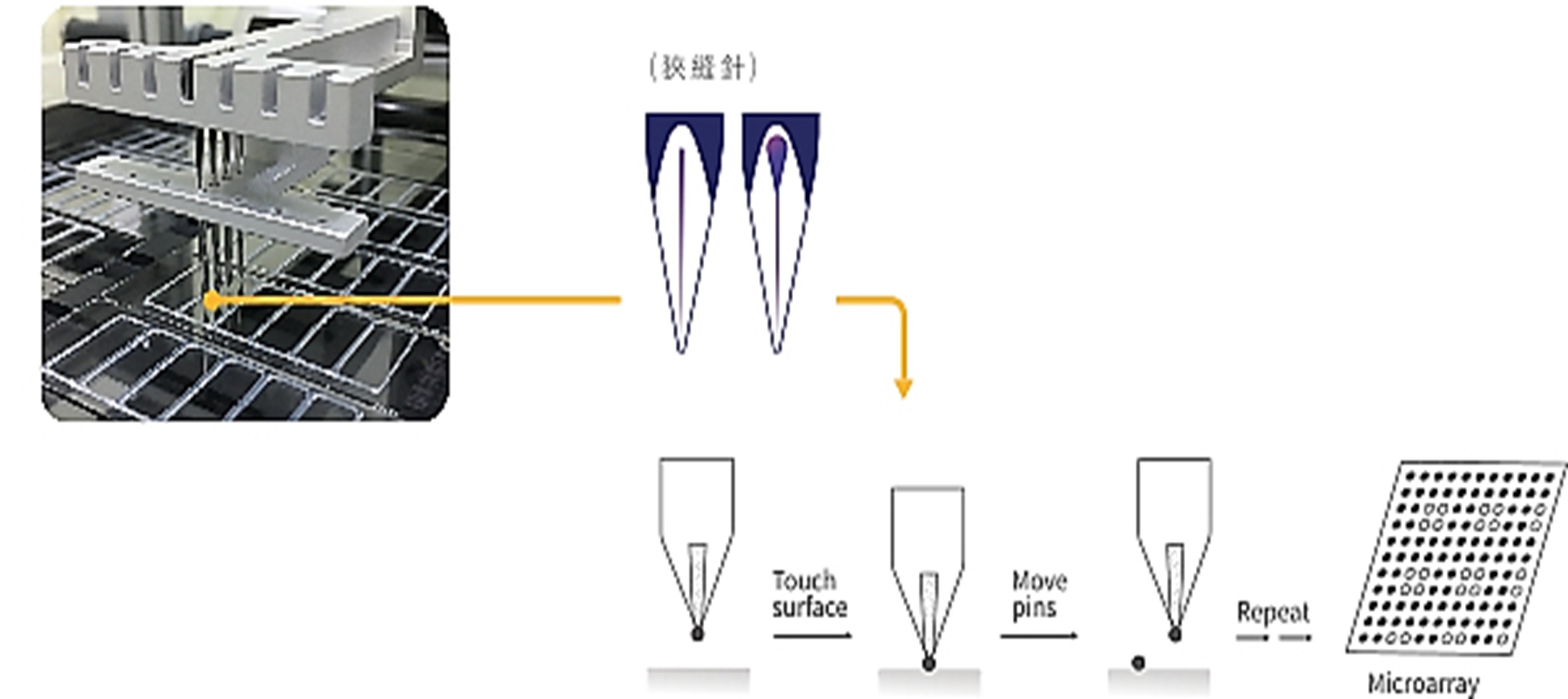
B. 蛋白質晶片點片系統(Spotting system)
凌越生醫過敏原蛋白質晶片主要以特製玻片為主體,採用人機介面點片系統,透過專屬的程式與自動化機械手臂系統,再依據不同蛋白質特性開發出相對應的點印配方,以高密度的方式點印於玻璃基材上,可在短時間內生產出品質穩定且同時偵測多項過敏原的蛋白質晶片。
C. 蛋白質晶片掃描系統(Scan system)
凌越生醫過敏原檢測試劑採用螢光方法,利用不同波長的雷射激發不同抗體上結合的螢光物質,以此區分不同的偵測項目;螢光檢測可偵測到picogram等級,訊號較其他檢測方法敏感且穩定。
相較於傳統的ELISA(Enzyme-linked immunosorbent assay)過敏原檢測分析,凌越生醫的過敏原檢測平台搭配半自動化掃描儀器及軟體分析,且採用螢光顯色方法,具有以下優勢:
a.微量化的反應環境:檢體需求低,對採血困難的族群友善。
b.穩定性與靈敏度佳:晶片上各抗原皆進行三重複之試驗,確保檢測之穩定性;同時可偵測至較低濃度(極限可低至pg等級)。
c.環保性與低成本:僅需少量試劑與耗材,減少資源使用。
d.效率高:多品項過敏原可同時檢測,同一試劑上也可同時偵測不同檢體,高通量節省時間。
e.擴充性:平台規格及檢測項目具有擴充之彈性,在增加過敏原品項的情況下,並不需要增加檢體量與試劑量,也提供了客製化的方便性。