EBSALERT SARS-CoV-2 (COVID-19) Test Kits (EUA Ended)
At the beginning of 2020, as the COVID-19 pandemic broke out, Excelsior Bio-System made the difficult decision to put all existing R&D projects on hold. Our entire team redirected their efforts toward the development of a COVID-19 antibody rapid test, hoping to contribute meaningfully to Taiwan and to the world.
By May, our dedication bore fruit—we became the first company in Taiwan to receive approval from the Ministry of Health and Welfare for a COVID-19 antibody rapid test. This was soon followed by CE certification and multiple international regulatory approvals.
COVID-19 is an acute respiratory disease caused by a novel coronavirus (SARS-CoV-2), known for its high transmissibility and significant mortality rate. It was declared a global pandemic by the World Health Organization (WHO). The virus, transmitted to humans through an unknown intermediary, spreads primarily through direct contact with infected secretions or respiratory droplets. Clinical symptoms are mostly respiratory in nature, including nasal congestion, runny nose, cough, and fever—typical of upper respiratory tract infections. Some patients may also experience gastrointestinal discomfort or loss of taste.
By May, our dedication bore fruit—we became the first company in Taiwan to receive approval from the Ministry of Health and Welfare for a COVID-19 antibody rapid test. This was soon followed by CE certification and multiple international regulatory approvals.
COVID-19 is an acute respiratory disease caused by a novel coronavirus (SARS-CoV-2), known for its high transmissibility and significant mortality rate. It was declared a global pandemic by the World Health Organization (WHO). The virus, transmitted to humans through an unknown intermediary, spreads primarily through direct contact with infected secretions or respiratory droplets. Clinical symptoms are mostly respiratory in nature, including nasal congestion, runny nose, cough, and fever—typical of upper respiratory tract infections. Some patients may also experience gastrointestinal discomfort or loss of taste.
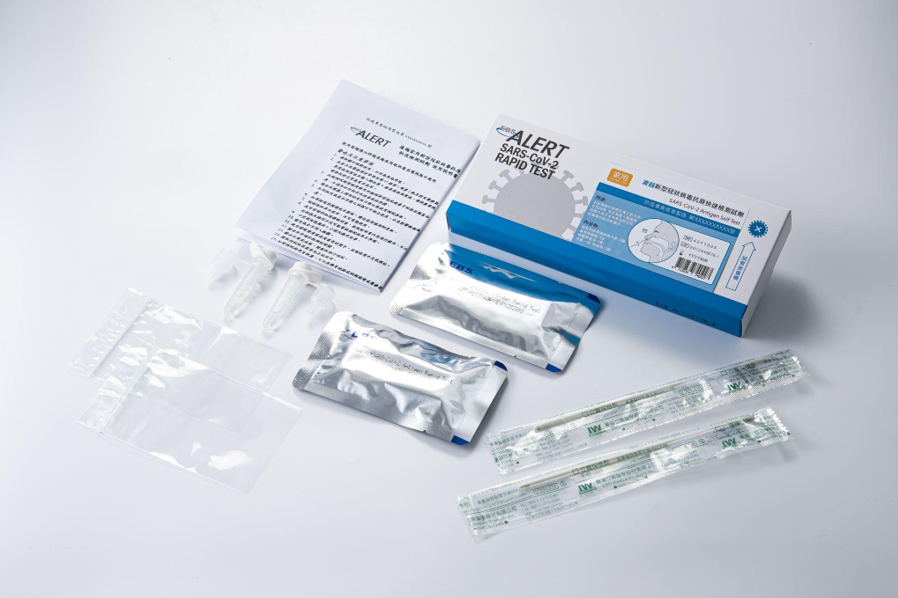
EBSALERT SARS-CoV-2 Antigen Rapid Test
EUA No. 1106033071

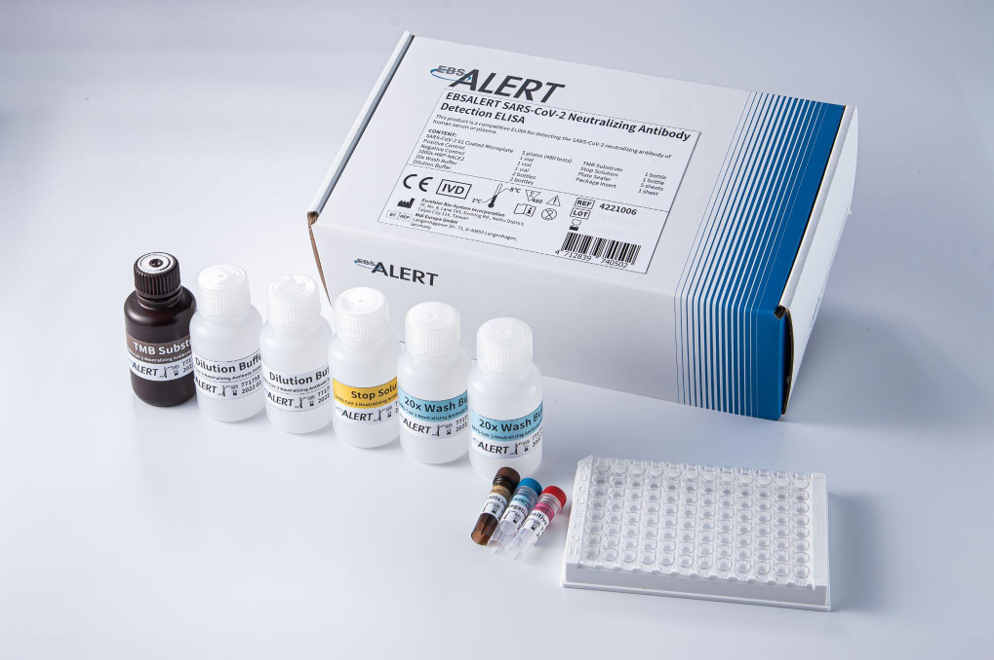
EBSALERT SARS-CoV-2 Neutralizing Antibody Detection ELISA
EUA No. 1106035513