Raw Material Preparation
A. Allergen Protein Purification Technology
Excelsior Bio-System (EBS) customizes our allergen protein antigen combinations based on local dietary habits and environmental exposure. By tailoring protein purification and extraction processes to the specific characteristics of different proteins, EBS produces high-quality whole protein antigen materials that align with everyday lifestyles.
B. Allergen Protein Material Quality Control
The stability of allergen detection reagents depends on the consistency of allergen protein raw materials. EBS has established a comprehensive quality management process to ensure that each batch meets high-quality standards:- Safe and Reliable Raw Material Sources:
- Material Stability:
- Allergen Activity Verification:
C. Substrate Evaluation
In microarray technology, selecting the right substrate directly impacts the stability and sensitivity of detection systems. EBS designs its development process to tightly align substrate properties with allergen detection needs. It tests a range of high-quality glass substrates, including options with high hydrophilicity, low background noise, and specialized functional coatings. This approach identifies the best substrate for achieving uniform allergen distribution and optimizing reaction efficiency.
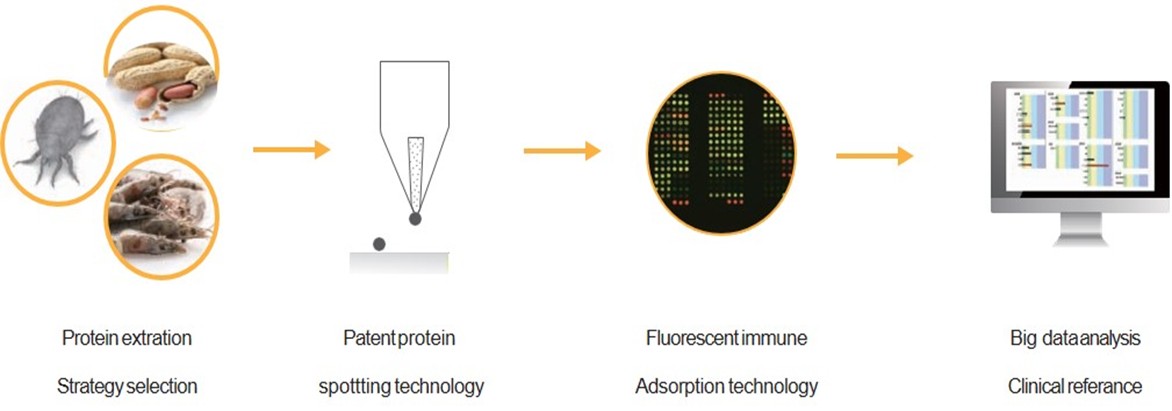
Microarray Protein Chip Technology
A. Microarray Commercialization
Using precision antigen spotting technology, extracted antigens are accurately deposited onto glass substrates. The specific antigen-antibody binding is paired with fluorescence immunoassay techniques to collect fluorescence signals triggered by laser excitation. The intensity of these signals is analyzed and translated into semi-quantitative clinical reports.
B. Protein Chip Spotting System
EBS’s allergen protein chips use glass slides as the primary substrate. With a human-machine interface spotting system and automated robotic arms, EBS develops customized spotting formulas based on protein characteristics. This enables high-density printing on glass substrates, producing stable protein chips capable of detecting multiple allergens in a short time.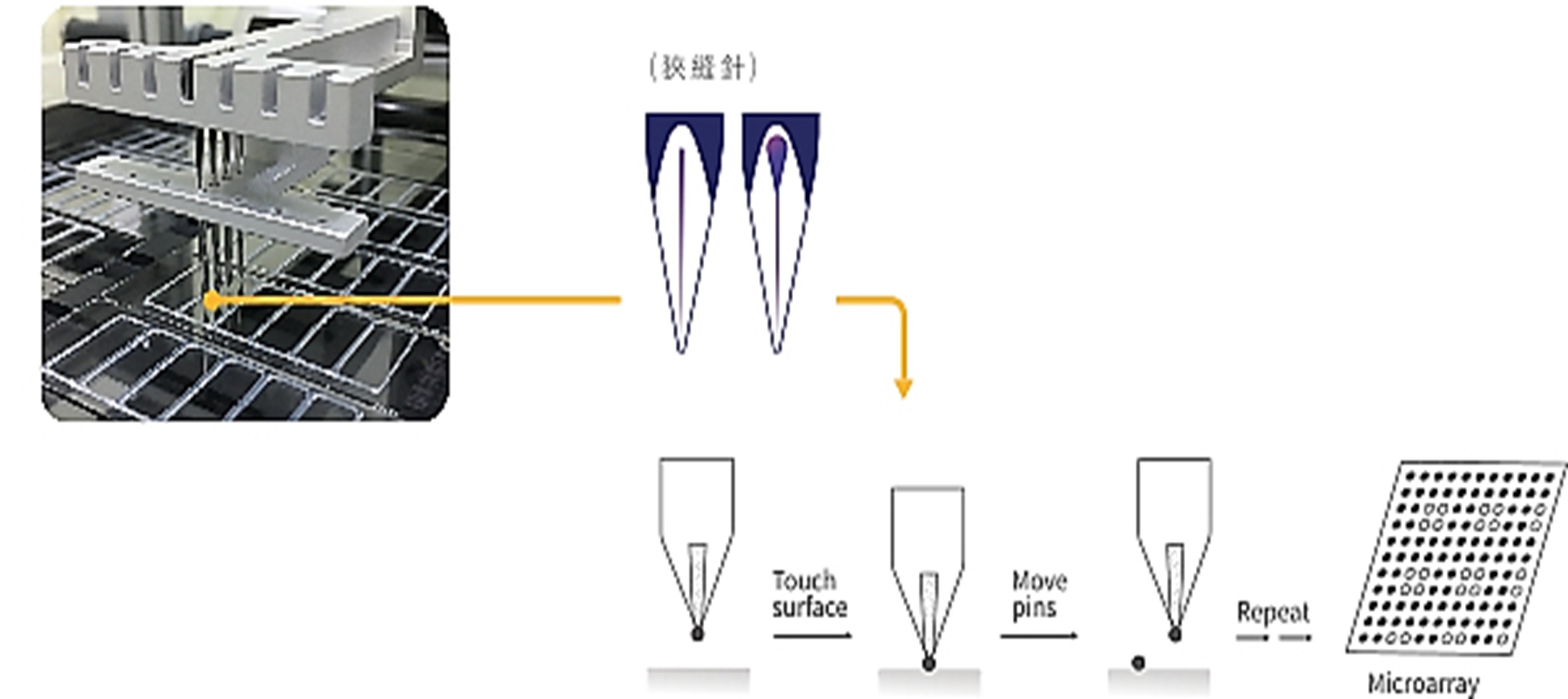
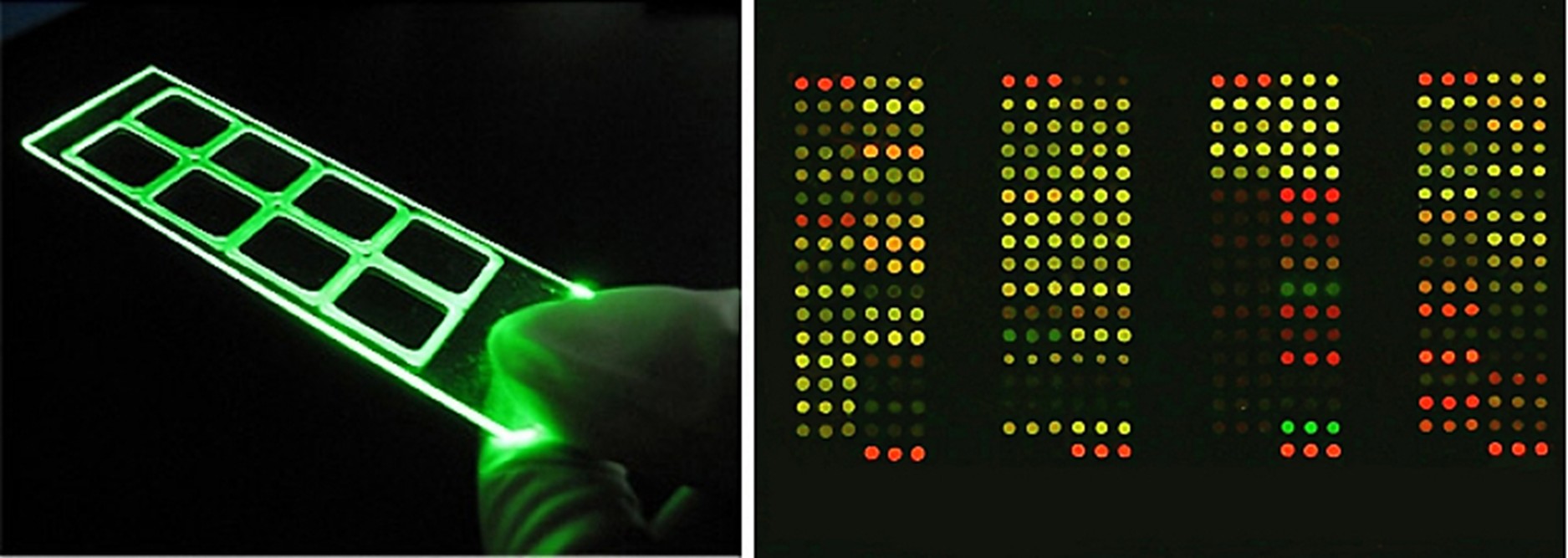
C. Protein Chip Scanning System
EBS’s allergen detection reagents utilize fluorescence-based methods. Lasers with different wavelengths excite fluorescent substances bound to other antibodies, enabling the differentiation of detection items. Fluorescence detection is highly sensitive and capable of detecting signals at the picogram level, offering greater sensitivity and stability than traditional ELISA (Enzyme-Linked Immunosorbent Assay) allergen detection.Advantages of EBS’s Allergen Detection Platform:
- Minimal Sample Requirement: A low sample volume is needed, making it suitable for populations with challenges in blood collection.
- Enhanced Stability and Sensitivity: Each antigen on the chip undergoes triple-replication tests to ensure detection stability, with sensitivity reaching picogram levels.
- Eco-Friendly and Cost-Effective: Requires minimal reagents and consumables, reducing resource usage.
- High Efficiency: Enables simultaneous detection of multiple allergens and different samples on a single chip, saving time with high throughput.